I had an hour to kill in Rutland today, and I walked a mile along the strip before I found this most awesome sign in the window of the most awesome Emporium Smoke Shop. They had more cigars than hookahs in there, but it was close. I just happened to have Ebert with me, and was graciously granted permission to capture spectra of the five different colors of discharge tubes.
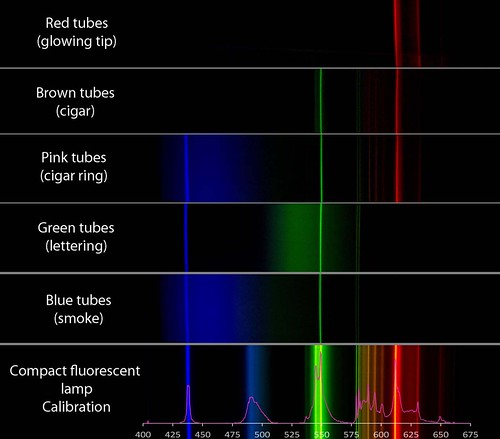
I am still puzzling out how the colors are produced, but I would say that mercury and europium are key, neon is not present, and colored glass tubes play a role. There may be some other tricks employed that I would love to know about. If Jim Stone (the owner of the shop) reads this, maybe he can comment below about the color of the tubes when the sign is turned off. Is the glass colored?

2 Comments
wow, cool -- i guess all mercury, huh? i suppose there aren't that many things that glow as bright as mercury, and if it gives you R, G and B, tinting the glass is a totally reasonable way to get colors. I do wonder if you can only get "cooler" colors since the mercury lines are so narrow. I don't mean bluer colors, but am thinking of the difference between a doped CFL and a basic mercury tube.
Is this a question? Click here to post it to the Questions page.
Reply to this comment...
Log in to comment
Mercury can account for the blue and green peaks, but not the red peaks. So all the tubes (except maybe the red one) have mercury. The red, pink, and brown tubes must include europium to produce the red lines at 580, 587, 593, 598, 611, and 630 nm.
So it appears that, like most lighting tubes, these contain a filler gas like argon with a little mercury in it. When a charge is applied, the excited mercury atoms release mostly UV light and a little visible light (the blue and green peaks at 436, 546, 577, and 579 nm). The red, pink, and brown tubes are lined on the inside with a europium compound which fluoresces orange and red when UV light excites it. The green and blue tubes do not appear to include europium.
The red tube shows no peaks from mercury, maybe because a red filter in the glass absorbs those wavelengths. There is probably mercury in there to produce the UV radiation which excites the europium.
The pink, green, and blue tubes have broad smears around particular mercury emission lines. I don't know how this is done. It might be a fluorescing phosphor, but it is not emitting discreet peaks the way most rare earths do. I don't think colored glass would have this effect. Mercury does not produce light at all of those wavelengths, so some other element must be producing it.
I have to remember to ask to see what the sign looks like when it is turned off.
Reply to this comment...
Log in to comment
Login to comment.