Oil testing kit Blog

Clips for the next spectrometer, pt 2
What I want to do
try out some clips I bought and figure how they fit in a new spectrometer design.
The clips
My frontrunner favorite was the tie clip-- but it was wobbly and doesn't stay in place well. The joint at the clip is just not firm.
The money clips were good. The longer, thinner black one was my favorite, and I expected as much.
Building a bench.
I painted one of the wider clips black, and cut small blocks of wood and painted them black. The big block for mounting the camera is 1cm x 1cm, and the ones for mounting the grating and slit are 1cm x 0.5cm
Then I made a small baffle out of black aluminum foil. I got spectra, but they were a little blurry. I need to modify the slit to be wider, and perhaps adjust the angle relationship between slit and camera. Currently, the grating is at 45 degrees and the camera at 60.
Conclusions
the long black clips are great, they stay in place, and combined with some black wooden blocks, would be perfect for attaching to the bench. I made the bench out of black foam board, it is alright, but a stiffer material would be better.
Follow related tags:
spectrometer oil-testing-kit

Clips for the next spectrometer
What I want to do
find and test a clip that works with the bench design @warren and I brainstormed for the next spectrometer design..
My attempt and results
We started with the clips we had-- binder clips--
But binderclips don't grip very well, and they are easy to knock around.
I explored a series of clips from the McMaster catalog, none satisfactory. The tall hole grip clamp with copper on it has these little tongs at the top that open up when the peg is pressed down, but it requires a custom pair of pliers to really work right, the alligator clips are great but suffer the same problem of the binder clips--too easy to bump.
I was most hopeful about these wire buckles. They are just the thing-- cheap, simple-- except they require a pair of pliers to open up. Too bad-- they are exceptionally nice and simple.
At the same time, I also ordered some anodized black aluminum right angle stock as the mount for our components. Checking if it was "infrablack" (black in the infrared) with an Infragram Point & Shoot, I found that it was more of a grey.
So in otherwords, none of the clips looked any good and the easiest right angle material didn't work either. back to the drawing board.
Next steps
I thought back to the binder clips-- maybe we could use them, but instead of grabbing everything together, use them to hold two grippy surfaces together that would hold the mount points in place. Here I'm imagining two rulers with grippy backs holdinig popsicle sticks in place with little mounting blocks on the popsicle sticks. This seems too complicated.
But then me and @warren were on the phone together banging our heads against online catalogs wondering how we were going to find the right clip when Google AdWords asked me "Are you looking for deals on mongraphed money clips?" Why no, Google, I wasn't, until you suggested it!
Money clips look kind of awesome. We're seeing them priced at 0.30-0.80 straight from a factory, and have seen a few black ones. I ordered a bunch on Amazon for too much money to try out.
I'm imagining we mount little blocks on the money clips, then they can be clipped at any angle to the bench. By printing markings on the bench, we invite people to make changes. If the bench has a little "give" to its surface the money clips should stick in place pretty well.
I also ordered some tie clips. and discovered the existence of a thing called the broccoli wad (youtube). To be continued...
Follow related tags:
spectrometer oil-testing-kit

Ultra micro cuvette tests, UV LED and low-temperature fluorescence
What I want to do
Just posting a variety of small tests I've done over the past few days, based a bit on the literature I've been looking through recently (this, this, and this).
Temperature and fluorescence
I'd read that temperature could affect intensity of fluorescence (Carstea, 2012), and since we're really trying to get fluorescence to be brighter, i tried preparing 3 identical samples of crude oil I ordered online, one drop each diluted in 1/4 oz of mineral oil:
That turned out to be a lot of oil, and the laser didn't really make it evenly through multiple bottles of it, which kind of ruined my experiment:
However, I'm really curious -- this was just to the naked eye, and I haven't validated with a spectrometer at all, but it seemed to me that the color of the cold (middle) cuvette was a bit more blue. Hmm. Not at all definitively, but I would love to try scanning this with a more dilute set of samples. Maybe it's an example of fluorescence saturation, which I read about in this article by Patsayeva (2000).
Ultra micro cuvettes
As a followup to this discussion on sample containers, I also tried out some new round-topped "ultra micro" cuvettes, which both reduce the amount of sample needed (they narrow to 2mm wide at the bottom) and since they have round caps, we hope they'll seal very well, unlike the square topped ones which leak. I'd read here and here that since the sample itself can absorb/block/filter out some wavelengths, it's best to use as thin and minimal a sample as possible, so the light emitted doesn't have to travel through more of the sample before reaching the camera.
I filled a couple with olive oil and one with the not-quite-dilute-enough crude oil from the temperature test above, and shot a laser through them:
Not bad! Also, the higher concentration doesn't seem to be a problem with such a thin (2mm) layer of sample. And it only took about 10 drops before the narrow part of the cuvette was full.
LEDs
I also tried using a big UV LED, which is either 385 nanometer or 395 nanometers -- I can't remember but will dig up the packaging again. It worked OK, although it wasn't that bright. Maybe it needs more power... not sure. I used 2 AA batteries, but I think it called for 3.5-4.5v. Still, if we could get this bright enough, it'd be much more compact than the big laser pointer.
Note the pink fluorescence from the olive oil vs. the blue from the crude oil sample. Cool!
Questions and next steps
One big question is: do the round-topped cuvettes seal? I've left them upside down and on their sides on a piece of cardboard and will be watching them for a day or two. Then I'll put them in my backpack and carry them around town wrapped in a towel and a ziploc bag for another couple days. We'll see, but the seal seems pretty good and no leaks yet after a couple hours! I should also try filling them with just mineral oil and shipping them somewhere.
If they work, they're <$1 each, and compact and lightweight!
Follow related tags:
spectrometer uv temperature oil

Continued literature review for the Oil Testing Kit
What I want to do
I've been reading and annotating a collection of formal research papers on fluorescence spectroscopy -- an in particular, laser induced fluorescence (LIF) spectroscopy, in order to connect our work with the Oil Testing Kit with existing literature, as well as to improve and support our techniques.
I'm going to start collecting them on this wiki page as well: http://publiclab.org/wiki/oil-testing-kit-literature where I'll link from to summaries and excerpted diagrams such as I'm doing in this post.
My attempt and results
I took brief notes on each one, and will excerpt illustrations and diagrams where it's helpful. Mostly I focused on their relationship with the techniques we're using or hoping to use.
Laser spectroscopy of mineral oils on the water surface
Patsayeva, S., et al. "Laser spectroscopy of mineral oils on the water surface." EARSeL eProceedings 1.1 (2000): 106-114. (PDF, Google Scholar)
- in-situ, of oil sheens, with 308 and 355 nanometer lasers (well below our 405nm)
- explored different kinds of spectroscopy, including lifetime decay (fading over a few nanoseconds) and "fluorescence saturation"
- showed normalizing to peak height -- peak of fluorescence, not laser -- in comparing different sheen thicknesses of same oil (very important -- see figure below and this note on normalization)
- "fluorescence saturation" - shining so much light in that light generating processes for some colors are limited by "slower emission kinetics" -- in particular red -- and there is a shift of the emission (fluorescence) peak towards the shorter, blue end of the spectrum. The amount of shift depended on the weight, or type, of the oil, and humic acid (not sure if this is related to plant matter?) shifted, interestingly, towards the red end. Could this be used to distinguish organic matter vs. fluorescence from petroluem, or is that not what "humic" means? (see below; the answer is yes)
Measuring of water and soil contamination with oil components using laser-induced fluorescence transmitted through optical fibers
Moise, N., Aurelia Vasile, and Mihail-Lucian Pascu. "Measuring of water and soil contamination with oil components using laser-induced fluorescence transmitted through optical fibers." ROMOPTP'94: 4th Conference on Optics. International Society for Optics and Photonics, 1995. (Google Scholar)
- nitrogen pulsed laser at 337.1nm (well below our 405nm), looking at both laser induced fluorescence (LIF/LIFS) and fluorescence lifetime/decay
- nice diversity of curve/peak shapes between 420-480nm (see figure below)
- discussion of crude absorbing strongly under 400nm, so need for shorter optical lengths ~1mm instead of 5-10mm (related to sample container discussion)
- experimentally derived emission maxima for Black Sea "East Swan" crude at 460nm, diesel oil at 438nm, and kerosene at 408nm in various mixes with soil -- these are in the visible range, so we could detect them if we could generate them with our 405 nm laser.
Field performance of a laser fluorosensor for the detection of oil spills
O’neil, R. A., L. Buja-Bijunas, and D. M. Rayner. "Field performance of a laser fluorosensor for the detection of oil spills." Applied Optics 19.6 (1980): 863-870. (Google Scholar)
- airborne, remote detection from a distance (a telescope pointed at the same point as a laser)
- tested in a dye spill vs. two crude oil spills, vs. background ocean water
- 380-700nm range sensing -- visible range and within our spectrometer's abilities
- UV filter removes laser backscatter, so you don't see a spike where the UV is
- 337nm exitation (nitrogen laser) -- well below our 405nm laser.
- plot of flight path vs. intensity at 20nm intervals, nice (see below)
Fiber-optic laser-induced fluorescence probe for the detection of environmental pollutants
Bublitz, J., et al. "Fiber-optic laser-induced fluorescence probe for the detection of environmental pollutants." Applied optics 34.18 (1995): 3223-3233. (Google Scholar)
- 337nm laser
- studied oil and sewer contamination, and a gas station
- organic matter fluoresced at 350-600, but up to 10mg/L addition of engine oil did not significantly change intensity -- so for small amounts of oil, organic matter could be a false positive
- looked at time-resolved laser induced fluorescence (LIF), which means brightness decay over time, did help to quantify hydrocarbons, as measured in <100 nanoseconds (way shorter than we can measure with a webcam)
- humic acid is indeed from humic substances, and shows fluorescence between 350-600nm
Fluorescence spectroscopy of polynuclear aromatic compounds in environmental monitoring
Kumke, M. U., H-G. Löhmannsröben, and Th Roch. "Fluorescence spectroscopy of polynuclear aromatic compounds in environmental monitoring." Journal of Fluorescence 5.2 (1995): 139-152. (Google Scholar)
- discussion of effects of polynuclear aromatic compounds (PAC, related to PAH), on human health
- "soil cleanup threshold" published by the World Health Organization and Dutch regulations: >200mg/kg
- "natural background" of PACs in rural soils estimated at <0.1mg/kg, highly contaminated sites >400mg/kg
- explains uses of in-situ monitoring: interest in high sensitivity, selectivity, discrimination from background environmental signals, speed of procedure, nonintrusive remote measurement at good spatial resolution, compact and rugged for field use
Monitoring and assessment of toxic metals in Gulf War oil spill contaminated soil using laser-induced breakdown spectroscopy
Hussain, T., and M. A. Gondal. "Monitoring and assessment of toxic metals in Gulf War oil spill contaminated soil using laser-induced breakdown spectroscopy." Environmental monitoring and assessment 136.1-3 (2008): 391-399. (Google Scholar)
This one wasn't really related to the Oil Testing Kit, and is a different kind of spectrometry, but since folks have been interested in detecting heavy metals, I thought we ought to look into it sometime. I think a ~1100nm laser (which is what a green laser is, with a frequency doubling crystal on the front) could be found strong enough to make sparks...?
- firing 1 joule of 1064nm laser at a compressed pellet of solid sample to detect aluminum, magnesium, calcium, chromium, titanium, strontium, iron, barium, sodium, potassium, zirconium, and vanadium
- it generates sparks and plasma, and detected peaks in the 390-650nm range
- good citations, worth another look
Detection of metals in the environment using a portable laser-induced breakdown spectroscopy instrument
Yamamoto, Karen Y., et al. "Detection of metals in the environment using a portable laser-induced breakdown spectroscopy instrument." Applied spectroscopy 50.2 (1996): 222-233. (Google Scholar)
Same deal; unrelated but really cool way to detect metals!
- uses a 15kg instrument, 46x33x24cm, 115v AC
- Ba, Be, Pb, and Sr in soils
- Pb in paint, Be and Pb particles on filters
- ppm detection limits in soils: 265 Ba, 9.3 Be, 298 Pb, 42 Sr
- 8000ppm for Pb in paint, 0.052mg/cm2, but using 220.35nm Pb(II) line instead of stronger 405.78nm line
- detection limit for particles on filters was 5.6ug/cm2
Questions and next steps
More reading and summarizing! All of this led me to many more papers. But it also brought lots of insights and things we should try out. It refocused attention on beam path through the sample -- inspiring some of the nanodrop and narrow beampath containers in the other note I published today, and had encouraging details about fluorescence data in the visible range where our device can work. Also we learned a bit about humic fluorescence from plant matter, and to watch out for it messing with our results! In particular, the article near the top (and the lead image) discussed "fluorescence saturation" and that humic fluorescence has a red shift when you hit it with "lots" of light, whereas heavier petroleums have a blue shift. Among other things we learned that fluorescence lifetime (how it fades over time) is a great technique for distinguishing oils, but happens too fast (<100 nanoseconds) for us to be able to use it. Maybe the Photosynq could do it?
Lots of papers talked about how great synchronous scanning fluorescence spectroscopy (SFS) is, but that'd require some major advances over our current design. I did have an interesting idea about stacking 2 spectrometers, one at 90 degrees from the other, and using the first to generate differentiated light along the slit of the second, resulting in a 2D matrix of exitation/emission data, but getting enough light into the sample to actually produce fluorescence would be quite difficult. Worth a try someday!
Why I'm interested
We'd really like to situate the Oil Testing Kit better in the scientific literature, and this is also a great way to get new ideas for methodologies, etc.
Follow related tags:
spectrometer spectrum-matching fluorescence heavy-metals
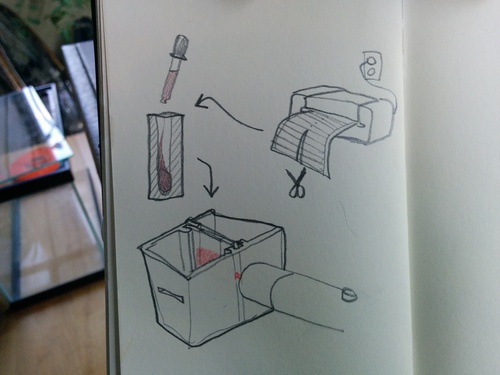
Sample container search
Note: Lots on sample containers is now collected on this page: https://publiclab.org/wiki/spectrometry-sampling
What I want to do
The square glass bottles we've used for fluorescence spectroscopy are sold out, and the nail polish bottles we've used are hard to get small enough and with flat sides. @mathew and I have been brainstorming some other options.
The literature review I've been doing mentions that it's best to let the laser pass through as little liquid as possible since the liquid can filter out the laser somewhat, and more importantly, the liquid can filter out the fluorescence emitted by the sample, like in this post by @eustatic:

Nanodrop and ultra micro cuvettes
@gaudi and @dusjagr's nanodrop research note, DIY Nanodrop and patent research by @mathew show a lot of options related to suspending a droplet somehow, either between two sheets of clear glass or plastic, or in some kind of hole:

One thing I worry about is making a mess -- it's great if we can completely seal the sample so it doesn't get toxic sludge all over the spectrometer, or your hands, and so that samples can be stored and transported.
"Ultra micro cuvettes" narrow at the bottom for a 1mm beam path (the thickness of the sample), but until I found this type with a round top, we were worried about leakage -- square-topped cuvettes have to be kept upright or they leak.
These have round caps, are plastic, UV-friendly, and $84 per hundred pack, $372 per 500: http://www.spectrecology.com/Disposable_Cuvettes.html or here on Amazon: http://www.amazon.com/BrandTech-759230-UV-Transparent-Disposable-Ultra-Micro/dp/B003ULPARY
These have a "chemical compatibility chart" here: http://www.brandtech.com/cuvette_comp.asp
It'd be too bad to use a specialty material, but we're already getting really particular about the jars, so they're not universally available either.
But Mathew and I talked today and we came up with the idea of something like a rigid Otter Pop, which could be made from plastic thermally sealed over a butter knife, more or less.
We could make them by sealing a roll of plastic (like a candy shop) every 1cm or so and cut it in half lengthwise, so they're open at one end. It'd be nice if we could use something rigid so they could slot in like a projector slide, and we could edge-light them with a laser. This needs to be tried out! @Mathew has a sandwich bag sealer :-)
So we have a lot of things to try out, now!
Follow related tags:
spectrometer fluorescence cuvettes sample-containers

Increasing brightness of fluorescence spectra
What I want to do
We had some trouble at the recent oil testing kit meetup in New Orleans in getting strong enough light into the spectrometer. @mathew and I have been discussing possible solutions but we'd talked about having a very wide slit, to sacrifice resolution for brightness, as a worst-case solution. I gave it a try today.
My attempt and results
https://spectralworkbench.org/analyze/spectrum/32235
It seems we can get enough light to break the 25% intensity line, even without modifying exposure or anything in the webcam. I did not use a reflector or anything, just a UV laser. But the sharpness of the UV peak was very poor.
For reference, I set it up in a temporary setup like this with a webcam, DVD, and laser added:
Questions and next steps
We have some other options to explore:
- reflectors behind the sample jar
- more laser!
- longer exposure, maybe through UVC USB controls
- a more sensitive camera
- a bigger lens
Why I'm interested
We need to both simplify and increase sensitivity in the Oil Testing Kit to get clear enough results to try matching. I hope we don't have to give up resolution, but this did seem to work all right, and maybe we'd be able to distinguish spectra even with reduced resolution.
Follow related tags:
spectrometer kits fluorescence desktop-spectrometer





























