Adapted from the Statistics for Action Air Quality Unit "Common Units" and generously shared with permission. You can access a printable version of this guide at sfa.terc.edu
Overview
This workshop guide will help articipants discuss, read, and practice using one or more units of measurement found in environmental science. Includes a fact sheet, and facilitator guide for each of the following units:
Metric prefixes (like kilo-, mega-, milli-, micro-)
Liters (L), milliliters (mL), and deciliters (dL)
Kilograms (kg), grams (g), milligrams (mg), and micrograms (µg)
Parts per million (ppm) and parts per billion (ppb)
Watts (W), kilowatts (kW), megawatt (MW), kilowatt-hours (kWh)
When to Use This Guide
You can use this guide before (or along with) a reading of technical documents or reports that refer to any of these units. Choose only the unit or units related to your community; don’t use all the fact sheets. You can use the fact sheets by themselves as handouts to supplement a meeting or other activity.
Notes for Facilitator The fact sheets use many analogies to help participants understand the units. These analogies can be unscientific and imprecise. They shouldn’t be used in a scientific way. However, they can be useful in making memorable messages or images for the media or the wider community
Order of Magnitude
“Order of magnitude” is a term often used in environmental work. It is a quick, general way to communicate the size or quantity of something. There are a few ways the term is used:
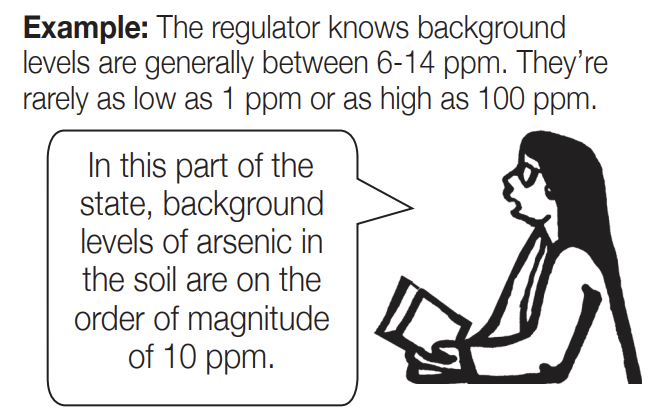
Estimating or Generalizing: When guessing the size of a number, people may not be able to be very specific, but they can guess whether it’s closer to 1, 10, 100, or 1,000, etc. (or 0.1, 0.01, 0.001, etc.) This “ballpark” estimate helps start a conversation, even without precise numbers.
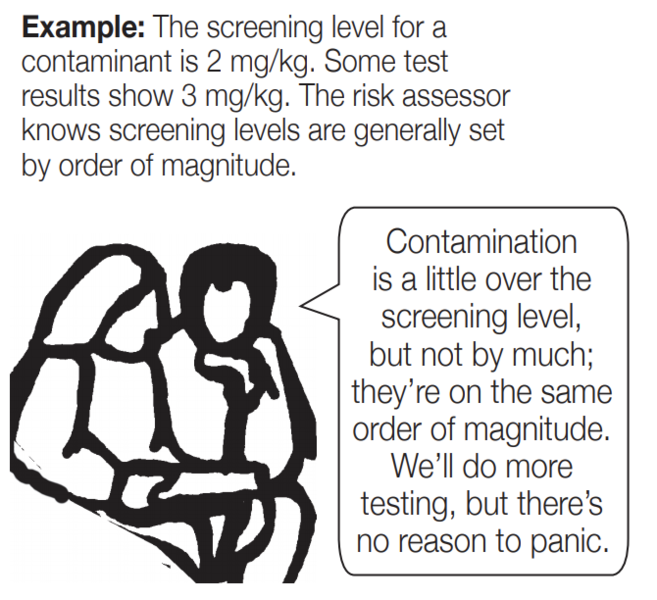
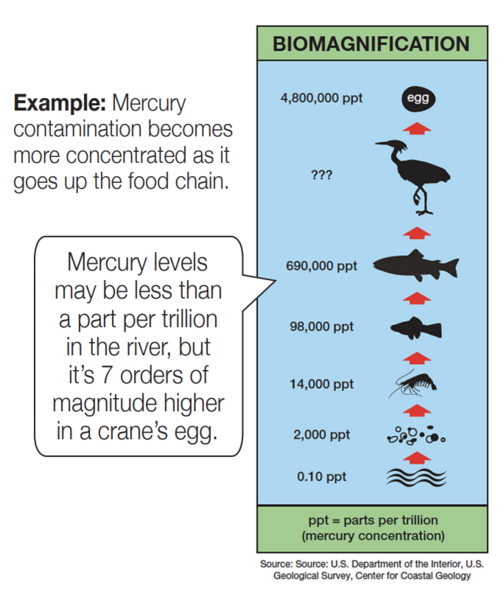
Comparing: When comparing two numbers quickly, the exact comparison might not be as important as the order of magnitude comparison. Health effects from contamination don’t change much with a small increase in contamination. Usually it takes an order of magnitude increase or decrease in contamination to cause a significant change in health effects.
Launch the Discussion. Remind or tell the group why you’re covering this topic (it came up at a previous meeting, it’s a key to understanding something the group has identified as a priority, etc.) Ask the group: Has anyone heard the phrase “order of magnitude” before? What do you think it is? Pass out the Fact Sheet. Review key points. Discuss with the group how it connects to their work.
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Activity 1: Ask the group to compare familiar things by order of magnitude. Make sure people say what aspect is being compared: Length? Area? Volume? Weight? Expense? Prompt with the comparisons below (don’t give an answer until participants have had a chance to guess.)
Height of an adult, and height of a toddler? (same order of magnitude)
Amount of water in a tablespoon, and amount of water in a gallon? (two)
Time in an hour, and time in a year? (four)
Weight of a bicycle, and weight of a car? (two)
Weight of a person, and weight of an elephant? (two)
Then, ask participants to come up with their own order of magnitude comparisons.
Activity 2: Find a common small object, like a post-it note or a dollar bill. Have the group figure out big it would be if it were one or two orders of magnitude larger. Mark how big it would be on the floor.
Metric Prefixes
Definition: The metric system uses standard prefixes with its units, to show changes in the order of magnitude.
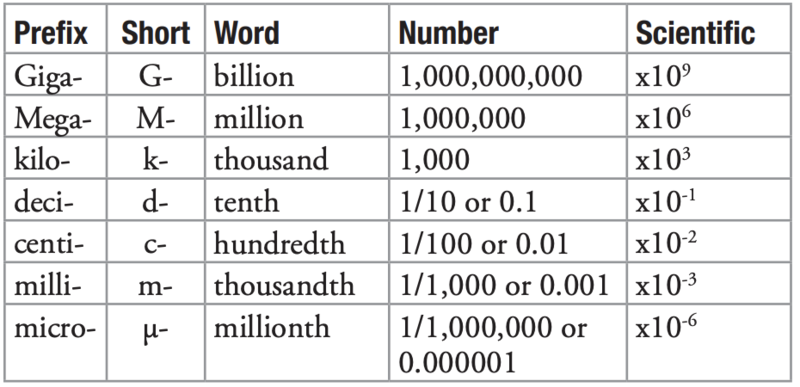
Examples: Grams, kilograms, and milligrams are different units. But they all measure mass; just on different orders of magnitude. For instance, a kilogram (kg) is 1,000 grams (g), 1,000,000 milligrams, and 1,000,000,000 micrograms. A milliliter (mL) is 1/1000 of a liter, or 0.001 L.
Uses: Technically, you can use any of these prefixes with any metric unit. In practice, some are common and others are rare. For example, it’s common to measure liquid in liters and milliliters, but deciliters are only used when talking about measuring toxins in human blood. Nobody uses kiloliters, but people working for public water systems might use megaliters or gigaliters. There is no special pattern; each profession has its own culture.
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Cubic Meter (m3)
Definition: A cubic meter (m3) is the space contained by a cube one meter on each side. It’s a measure of volume, equal to 1,000 liters, or 264 gallons. (A meter is 100 centimeters, the same as 39.4 inches, or a little longer than a yard.)
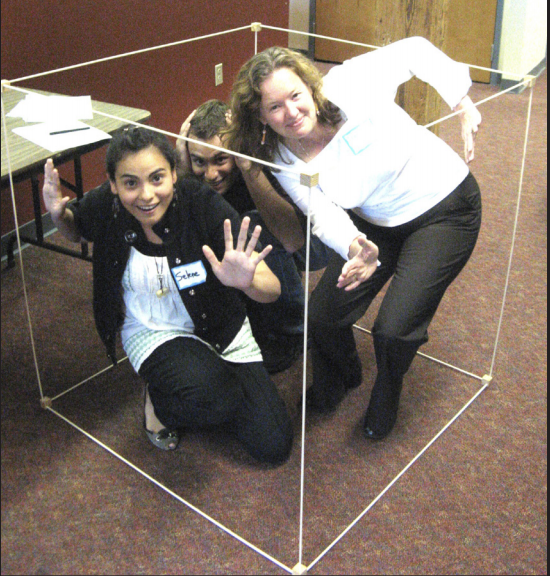
Uses: Cubic meters are used to measure a volume of air. Air contamination is usually measured in milligrams or micrograms of contamination per cubic meter (mg/m3 or µg/m3).
Examples: - Five 55-gallon barrels are about the same as a cubic meter.
The space underneath a 6' x 2½' table that is 2½' high is about a cubic meter.
A 12-foot storage pod holds about 21 cubic meters.
A typical refrigerator takes up about 1.5 cubic meters of space in a room (but holds less than that).
A typical adult male breathes a cubic meter of air every 2 hours.
Launch the Discussion: Remind or tell the group why you’re talking about cubic meters (it came up at a meeting, it’s a key to understanding something the group has identified as a priority, etc.) Ask the group:
Has anyone heard of a cubic meter before? It might be best to compare it to a standard 55-gallon steel drum or barrel - the kind used for oil or waste. (Confirm participants are familiar with these before continuing.) How many 55-gallon drums do you think are in a cubic meter? (Read the list and have participants vote, but don’t give an answer until they have had a chance to guess.)
Half of a 55-gallon drum?
A full one?
Two?
Five? (Answer: A cubic meter is a little less than five 55-gallon drums.)
Ten?

Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Activities
Build a cubic meter using 12 meter sticks. Alternatively or use 3 meter sticks and the corner of a room to define the outlines of a cubic meter.
Find something in the room that takes up about a cubic meter of space (or something like half or twice that size). Measure it out to confirm. Estimate: how many of those would fit in the room? Then measure the dimensions of the room to confirm.
How much lead (or any other contaminant) would it take to contaminate the air in the room above the air quality standard of 0.15 µg/m3 (or any other air quality standard?) Calculate the volume of the air in room in cubic meters. Use this Fact Sheet together with the Fact Sheet for kilograms and grams if needed.
Liters (L), milliliters (mL), and deciliters (dL)
Definition: A liter (L) is a measure of (usually liquid) volume. A cube 10 centimeters (cm) on each side is equal to a liter, though a liter can be any shape. A milliliter (mL or ml) is 1/1000 of a liter. It’s equal in volume to a cubic centimeter (cm3 or cc). A deciliter (dL) is 1/10 of a liter.
A “drop” is not an official measurement because actual drops of liquid come in many sizes. However, a general rule is that there are 20 “drops” in one mL.
Uses: Liters are the standard way to measure large amounts of liquid, especially water, in scientific laboratories. Water contamination is usually measured in miligrams or micrograms of the contaminant per liter of water (mg/L or µg/L). One liter of water (but not other liquids) has a mass of one kilogram (kg). This means that you can convert mg/L to “parts per million” and µg/L to “parts per billion” when describing water contamination. Can you find the million-to-one relationship in mg/L? Milliliters are used for smaller measures of liquid, like liquid medicine. One mL of water has a mass of one gram (g). Deciliters (dL) are used in giving blood test results, but aren’t common to see elsewhere.
Examples:
A liter is a little bigger than a quart. There are about 3.8 liters in a gallon.
The most common size for a large bottle of soda/pop is two liters, which holds a little less than a six-pack of 12-ounce cans.
A typical bottle of wine is 750 mL, or 3/4 of a liter.
A 12-cup coffee carafe is about 1.5 L.
There are 5 mL in one teaspoon.
One dL is a little less than half of a measuring cup.
Launch the Discussion: Remind or tell the group why you’re covering this topic (it came up at a previous meeting, it’s a key to understanding something the group has identified as a priority, etc.)
Ask the group: Has anyone heard of a liter before? What’s an example of a liter? Which do you think is closer to a liter?
A measuring cup? (about 1/4 of a liter)
A quart? (a quart is a little less than a liter)
A gallon? (3.8 liters)
Fact Sheet: Pass out the Fact Sheet. Review key points. Discuss with the group how it connects to their work.
Activities: Display several empty bottles or containers of different shapes and sizes, labeled A, B, C, etc. At least one should be a liter. If possible, include a 10 cm x 10 cm x 10 cm cube. Write down the capacity of each bottle, for your own notes. Remove or cover any labels on the bottles that say how much they hold. Have participants guess which one is a liter. Have a jug or pitcher of water handy so people can check their guesses and compare by pouring water from a smaller container into a larger one.
Kilograms (kg), grams (g), milligrams (mg), and micrograms (µg)
Definition: A gram (g) is a metric unit for a small amount of mass or weight. It’s equal to the weight of one cubic centimeter (or one milliliter) of water. A kilogram (kg) is 1,000 grams. A kg has the same weight as one liter of water. A milligram (mg) is one thousandth of a gram. There are one thousand mg in a g, and one million mg in a kg. A microgram (µg, ug, or Ug) is one millionth of a gram. There are one million µg in a g, and one billion µg in a kg.
Uses: In environmental science, these units are frequently used in relation to each other, because even a tiny amount of contamination in a large amount of soil, water, or air can be harmful. Soil contamination is often measured in mg or µg of contaminant per kg of soil sampled. Water contamination is measured in mg or µg of contaminant per liter of water. Air contamination is measured in mg or µg of contaminant per cubic meter of air.
Note:Because there are one million mg in a kg, the ratio mg/kg is sometimes expressed as “parts per million (ppm).” Similarly, µg/kg sometimes appears as “parts per billion (ppb).”
Examples:
A kg weighs about 2.2 pounds.
There are about 28 g in 1 ounce.
A 2-L bottle of soda/pop weighs 2 kg. A major league baseball bat weighs about 1 kg.
A dollar bill, a small paper clip, and a packet of artificial sweetener each weigh about 1 g.
Medical pills are often described in mg, but you shouldn’t use that as a way of understanding how big a mg is: A “200-mg” tablet of ibuprofen contains 200 mg of the drug, but it also contains fillers and coatings to help the drug release slowly. A whole 200-mg pill weighs much more than 200 mg.
One mg is almost too small to see. One grain of fine table sugar or salt might be about one mg. One µg is definitely too small to see without a microscope.

Note: The discussion and activities below focus on grams and kilograms. If your group is more interested in milligrams and micrograms, first do the activity below. Then, use the 1,000-to-1 relationship between kg and g to imagine the same relationship between g and mg, or mg and µg.
Launch the Discussion: Remind or tell the group why you’re talking about these units (it came up at a meeting, it’s a key to understanding something the group has identified as a priority, etc.) Ask the group: Kilograms: What’s an example of a kilogram? Which of these do you think is closer to a kilogram? (Read the list and have participants vote, but don’t give an answer until they have had a chance to guess.)
A deck of cards? (about 1/10 of a kilogram)
A baseball bat? (about 1kg)
A gallon of water? (3.8 kg)
A cinder block? (12 -14 kg)
Grams: What’s an example of a gram? Which do you think is closest to a gram? (Read the list and have participants vote, but don’t give an answer until they have had a chance to guess.)
A drop of water? (0.05 g)
A piece of copier paper? (about 4.5 g)
A packet of artificial sweetener? (about 1 g)
A tablespoon of sugar? (about 12 g)
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work. If the group is focused on mg and µg, try to put together statements like, “There are as many milligrams in a packet of sweetener as there are packets of sweetener in a baseball bat.”
Activities: Bring in a digital bathroom scale (for kg) and/or a sensitive digital kitchen scale (for grams).Bring in objects of various weights and invite participants to guess the weight in kilograms/ grams, and then weigh the objects to verify. Invite people to guess and check with objects in the room or in their pockets: Books, keys, wallets, water bottles, etc. For a special challenge, have participants guess which one is closest to 1 g or 1 kg.
Acres and Hectares
What is It?: An acre measures surface area. It’s equivalent to 43,560 square feet. There are 640 acres in one square mile. The acre was originally 22 yards by 220 yards, the amount of land that one person could plow in one day with one ox. But there is no standard shape for an acre; it is any shape with that area. In the metric system, the thing most similar to an acre is a hectare, which is 10,000 square meters, or a square measuring 100 meters x 100 meters. A hectare is about two and a half acres.
Uses: In the U.S., it’s common to measure large areas of land in acres. Plots of land for building houses are frequently described as fractions of acres (1/4 acres, 1/10 acre, etc.) Hectares are rarely used in the U.S., except when comparing to international measures. A quick internet search will tell you the area of most cities and states in acres or square miles.
Examples:

Launch the Discussion: Remind or tell the group why you’re covering this topic (it came up at a previous meeting, it’s a key to understanding something the group has identified as a priority, etc.)
Ask the group: What’s an example of an acre? Which do you think is closest to an acre? (Read the list and have participants vote, but don’t give an answer until they have had a chance to guess.)
A football field? (a little more than an acre)
An average Home Depot? (about 3 acres)
The area of Rhode Island? (776,957 acres)
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Activities: Use a tape measure to measure the floor of the room. Calculate the floor’s area in square feet. Alternatively, you can come with information about the area of a place everyone knows (like the local high school gym). Then calculate how many ‘floors’ of that room would make an acre.
Tons and Tonnes
Definition: One ton, also known as a “short ton,” is 2,000 pounds. One tonne, also known as a “metric tonne” or “metric ton” is 1,000 kg, or 2,204 pounds. One “long ton” is 2,240 pounds. This term is rarely used in the U.S. except in shipbuilding.
Uses: Tons and tonnes are usually used for very large weights of solid material, like waste going to a landfill, or coal going to a power plant. In the U.S., the word “ton” by itself usually means short tons. People who mean metric tonnes will usually specify that.
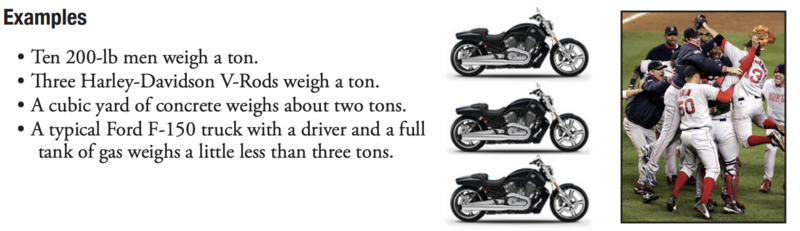
Launch the Discussion: Remind or tell the group why you’re covering this topic (it came up at a previous meeting, it’s a key to understanding something the group has identified as a priority, etc.)
Ask the group: Has anyone heard of a ton before? What’s an example of something that weighs a ton?Which do you think is closest to a ton?
A Harley-Davidson? (A Harley V-Rod is about 1/3 ton.)
A VW Bug? (1967 model was a little less than a ton. The 2012 model is 1.5 tons)
A Hummer 2? (Over 3 tons)
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Activities: Using a bathroom scale, weigh a few heavy objects in the room. Then calculate how many of each object would be needed to make a ton.
Parts per million (ppm) and parts per billion (ppb)
Definition: Parts per million (ppm) and parts per billion (ppb) show a relationship between two quantities that use the same units. A part per million could be one drop per one million drops, one gram per one million grams, etc. By definition, one ppm = 1,000 ppb. Ppm/ppb can be measured by weight (ppmw/ppbw) or by volume (ppmv/ppbv), depending on what’s being measured. Some documents will tell you if it’s weight or volume, other times you will need to figure it out yourself.
Uses: Ppm and ppb are often used like a percent, but for very small amounts. The word percent literally means “part per hundred”. So, 1% = 10,000 ppm = 10,000,000 ppb. For soil, mg/kg = ppm and µg/kg = ppb. This is always by weight. For water, mg/L = ppm and µg/L = ppb, if comparing by weight. These equations are not true in the rare cases where measuring ppm/ppb by liquid volume. For air, ppm and ppb are always by volume. There is no direct conversion between ppm/ppb and mg/m3 or µg/m3; it will depend on the density of the contaminant in the air, and on air temperature and pressure.
Other ways of thinking about parts per million:
1 drop of ink in a large kitchen sink (about 13 gallons).
One drop in the fuel tank of a mid-sized car
One inch in 16 miles
One minute in two years
One car in a line of traffic from Cleveland to San Fransisco
One penny in $10,000
Other ways of thinking about parts per billion: - one drop of ink in a large tanker truck (about 13,000 gallons) - 1 car in a line of cars that goes around the Earth 100 times - Three seconds out of a century - One penny in $10,000,000 - One grain of sand in a sand box

Launch the Discussion: Remind or tell the group why you’re covering this topic (it came up at a previous meeting, it’s a key to understanding something the group has identified as a priority, etc.)
Ask the group: Has anyone heard of a part per million before? What’s an example of a part per million? Which do you think is closest to a part per million? Is it like one drop of ink in:
A cup of water? (1 part in 4,730, or 211 ppm)
A gallon of water? (1 part in 75,700, or 13 ppm)
A large kitchen sink? (about 1 ppm)
An olympic-sized swimming pool? (1 part in 5,000,000,000, or 0.005 ppm, or 5 ppb)
Ask the group: Has anyone heard of a part per billion before? What’s an example of a part per billion? Which do you think is closest to a part per billion? Is it like one drop of ink in:
A large kitchen sink? (about 1 ppm, or 1,000 ppb)
A bathtub? (1 part in 3,180,000, or 315 ppb)
An olympic-sized swimming pool? (about 5 ppb. This is the closest answer, but the most accurate would be one drop spread across five olympic swimming pools)
Lake Erie? (1 part in 1x1018, or one billionth of a part per billion)
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Activities: In the SfA activity Converting Between Units, participants practice converting parts per million or parts per billion to either mg/kg and µg/kg (for soil) or mg/L and µg/L (for water).
Watts (W), kilowatts (kW), Megawatts (MW), kilowatt-hours (kWh), Megawatt-hours (mWh), and Million-Megawatt-hours (MMWh)
Definition: One watt (W) is a measure of electrical power, which is the amount of energy an electric device uses (or produces) per second. A kilowatt (kW) is 1,000 W, and a megawatt is 1,000,000 W. A kilowatt-hour (kWh) is the amount of energy used by using one kilowatt of power for one hour. A megawatt-hour (MWh) is the amount of energy used by using one megawatt of power for one hour. One million-megawatthour (MMWh) is one million megawatt hours.
Uses: Household appliances are rated in watts or kilowatts, to show how much power they consume when in use. An electricity bill shows how many kilowatt-hours of energy were used. Power plants are rated in megawatts, to describe how much power they can produce. Megawatt-hours and million-megawatt hours might be used to describe how much energy a power plant produced in a year, or how much energy a whole city or state used in a year. Sometimes an amount of power plant fuel (coal, oil, natural gas, biomass, nuclear) will be described in MWh. This is an estimate of the amount of heat energy it can create if burned. Not all of that energy can be converted into electrical energy
Examples:
Old-style incandescent light bulbs use 60W or 100W of power.
A hot-air hair dryer or a toaster oven might use about 2 kW of power.
One kW is about 1.34 horsepower.
A major FM radio station transmitter broadcasts 50 kW of radio signal.
A small power plant might produce less than 100 MW for about 50,000 homes. Large power plants might produce 500-1,000 MW.
Fact Sheet: Pass out the Fact Sheet. Discuss with the group how it connects to their work.
Originally published by TERC in 2014 with support from the National Science Foundation and shared with permission. Any materials posted on Public Lab are not endorsed by TERC or NSF and do not necessarily represent the views of either organization. Images courtesy of the Rini Templeton estate.
0 Comments
Login to comment.