Cover Image: Photo by Ken-ichi Ueda, Flickr, CC BY-NC 2.0
A 2001 paper titled "Photosynthesis, Chlorophyll Integrity, and Spectral Reflectance in Lichens Exposed to Air Pollution" identifies the relationship between elemental concentrations in Ramalina lacera lichen (pollutants the lichen has absorbed from the air), and how well the lichen performs photosynthesis. This study is interesting because it is one of few studies that examines spectral reflectance (what wavelengths a specimen reflects) in lichen bioindicators, and it can serve as a "proof-of-concept" for a method that can be implemented with community science tools. More specifically, this method would involve using near-infrared camera or a spectrometer to calculate the normalized difference vegetation index (NDVI), a measure of vegetation health, of lichen specimens, which could then be transformed into information about pollutants in the air.
This note summarizes the methods and results from this study and offers ideas for modification for a community science application.
METHODS
The study determined elemental concentration of lichen via inductively coupled plasma atomic emission spectrometry (ICP--AES), which is a common approach for lichen elemental analysis.
The study also measured various indicators of photosynthetic ability, including the following:
- Chlorophyll content: Measured after extraction with a solvent
- Integrity of chlorophyll: Chlorophyll 'a' and phaeophytin 'a' concentrations were determined with a spectrophotometer , and the ratio is a proxy for chlorophyll integrity because phaeophytin can interfere with photosynthesis. Read more here
- Chlorophyll Fluorescence: Chlorophyll fluorescence---a practical guide has a good overview of how this analysis works, but fundamentally, the more fluorescence (emitted light), the lower the ability of the lichen to photosynthesize. This is measured with a fluorometer.
- Net Photosynthetic Rate: CO2 exchange rate of the lichen is measured with a infrared gas analyzer.
- Spectral Reflectance and NDVI: The average reflectance of the lichen in the near infrared (NIR) range, wavelengths between 800-1000nm, with a Li-Cor LI 1800 field spectrometer.
RESULTS
The study found a positive correlation between photosynthetic rate, chlorophyll content, NDVI, and K (potassium) content. It also found an inverse correlation between NDVI and Ba, Cr, Cu, and Ni content, which is in line with studies showing that heavy metals like Cu and Pb have detrimental impacts on lichen health and photosynthetic ability. The following quotes from the paper summarize related, promising work comparing NDVI with elemental concentrations:
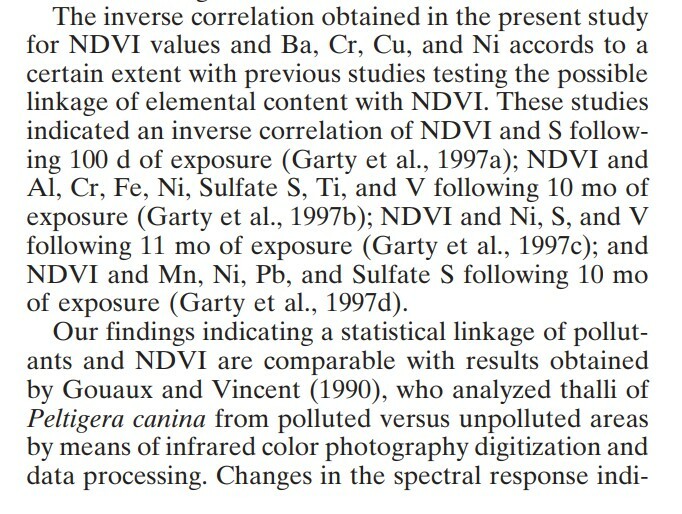
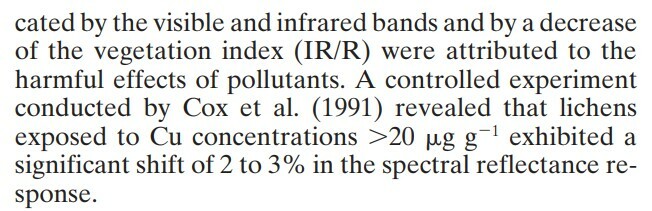
NDVI, Lichens, and Community Science
This paper and related research indicate that community science methods using NIR imaging (using a modified camera or off-the-shelf NIR camera) and/or spectrometry with lichen to calculate NDVI can be a viable proxy for relative air pollutant concentration. Possible methods include:
- Comparing NDVI for the same species of lichen sampled in various locations to air monitor data to develop a response curve for pollutants of interest, then use the response curve to estimate level of pollutants for lichen sampled in areas without a reference monitor.
- Comparing compare the NDVI of lichen sampled in a "clean" air area with that of lichen sampled near polluted areas to determine the relative severity of the pollution.
Note that NDVI analysis will not provide measurements of pollutants in the air, rather, it is most useful to compare relative lichen health within a geographic region with similar weather, rain, and sun conditions. Care should be taken to minimize non-air quality-related effects that would impact the NDVI, including, but not limited to, differences in lichen species, temperature, precipitation, and insolation. Additional research quantifying the impact of these and other non pollution-related parameters is needed to adjust for these effects and draw out only the pollution-related effects on relative NDVI.
Garty, J. & Tamir, O & Hassid, I & Eshel, Amram & Cohen, Y & Karnieli, Arnon & Orlovsky, L... (2001). Photosynthesis, Chlorophyll Integrity, and Spectral Reflectance in Lichens Exposed to Air Pollution. Journal of environmental quality. 30. 884-93. 10.2134/jeq2001.303884x.

25 Comments
Quite interesting. It will take several more readings before it's understood.
Thank you for reading! Let me know if there's anything I can help clarify
Reply to this comment...
Log in to comment
Working my way through the paper. One of the things the original talks about is " Extraction by immersion in DMSO" and "OD435/OD415 nm". This is equivalent to absorbance at these two wavelengths. The paper references "Pigment Extraction from lichens with DMSO and estimation of chlorophyll degradation" by R.Ronen, Margalith Galun ,Environmental and Experimental Botany, Volume 24, Issue 3, August 1984, Pages 239-245. I got the summary. Unfortunately, it doesn't give extraction conditions. Any help is appreciated.
I have access to the paper you're talking about--here is a snippet of the section you may be most interested in:
Let me check if I'm allowed to download the whole paper and share it with you.
Is this a question? Click here to post it to the Questions page.
Reply to this comment...
Log in to comment
Thank you. That's what I was after. Much appreciated!!!
Reply to this comment...
Log in to comment
Ok, the parameters are listed. Is there an example of a study set up?
Is this a question? Click here to post it to the Questions page.
I don't know of a community science project using the pigment extraction method since there are some specialized steps and equipment, but we're curious to see if you or anyone has ideas for a more DIY-able version!
Still have to do more work. But the acetone extract seems like something that might be doable in a home lab type situation. The acetone extract is where I'm going. Nail polish remover is something like 70% acetone. And no bad extraction conditions are required. Magnesium carbonate is available in the over the counter section of most pharmacies. The public lab spectrometer should be able to cover the narrow range needed (say 425 nm area) without much problem. The question is the centrifuge.
One word of warning. Solvents ( like acetone) and plastic cuvettes do not usually go together. The worst case is the plastic melts, but the plastic will also smear, which also makes the cuvette unusable. So the other choices are glass of quartz. Quartz is the better choice, since it is good through the entire Uv/vis, but you will usually pay for the honor. Glass should go below 400 nm without any problems, but double check the vendor specks to be sure.
Ok. Trying to stay as close to duplicate the original paper as closely as possible. R.la cera isn't in this area. But other closely related lichen supposedly do. First shot is the Fruticose lichens. No one knows of any close by, but have a lot more work to do on that road. Found an interesting video on YouTube "Using lichens to determine air quality" by the naturalist. As for method development. Looking for possible alternatives to the centrifuge. Possibly, disposable hplc filters. Need some help here. Why are the lichen stored in the dark during the extraction?
Is this a question? Click here to post it to the Questions page.
Was checking one of the online vendors. Found new centrifuges for $70. Industrial quality centrifuge costs were much higher, but who knows. If you are interested in making a hand powered centrifuge, try BioRxiv. The article is titled "A 3D printed hand powered centrifuge for molecular biology". It will handle up to about 2 ml of sample. And for a review of this type of centrifuge see YouTube "Recreating Paperfuge: The hand powered centrifuge" by Qosmos Chen. With a normal cuvette, at least 2 ml is needed. Even then, the cuvette will not be full.
As for the mortar and pestle, there are many options. Some mortar and pestles are very expensive, but they don't have to be. They should be easy to clean and non porous. And the pestle should be easy to grasp. Usually , we would raid the stopper drawer for a single hole stopper. Then, the stopper would go over the top of the pestle. It made it easier for some of the common grinding operations that go on in the lab.
Another, more current option , is to use a coffee grinder. With the temperature issues this material shows, probably using the frozen state would be good, if possible. Throw the samples in the freezer the night before, then prep them in the coffee grinder. Make sure the minimum amount of time necessary is used. Don't know if it would work. But it sure would save time.
A test of this method might be to use lawn clippings and run it through the procedure. The idea is to extract chlorophyll, which should be present in lawn clippings ( although probably a different kind of chlorophyll). Will be writing up a draft test method over the next few days.
If you are using the public lab spectrometer, a light source for around 400 nm is needed. It looks like there are some blue or violet led bulbs/ flashlights that cover this range. Might be a good place to start. The other option is the lines in the 660 nm range. Which should also be doable with leds.
There are 3 in 1 flashlights ( blue, white, and red) that would be good for this application- as long as the color was more like purple instead of blue. The flashlight would be able to switch between the ends of the spectrum, for each individual anaysis, as well as an overall scan. Blue is something like 445 nm to 490 nm. The area of chlorophyll peaks needs is more like 415 nm to 435 nm. ( if you down the standard ROYGBIV, this is indigo or violet). Also looking for light bulbs filling this requirement. There are a few. Will keep looking.
Looking into the 660 nm lines. It's not that LEDs don't exist for 425 nm region. They do. But the ones in the catalogs seemed expensive. Going down the 660 nm road, now.
Best bet- use an old fashioned tungsten lamp. Not a LED claiming to be a tungsten lamp. An LED has a narrow bandwidth. An old fashioned tungsten lamp should have a good bandwidth at the low end of the visible spectrum. Many tungsten lamps are still listed outside of the non-standard sizes.
The tungsten lamp remarks only apply to the 660 nm red lines.
Reply to this comment...
Log in to comment
Out of curiosity only.
Reply to this comment...
Log in to comment
I went to ohiomosslichen.org/ to see what's in the ohio area. Didn't seem like there was anything directly comparable to the lichens in the paper. What do you think?
Is this a question? Click here to post it to the Questions page.
There's some work needed to modify this research protocol so it can be done in regions like Ohio that might not have the exact species listed in the paper. A good place to start is this US Forest Service table that indicates lichen sensitivity to certain pollutants, and you can use it to get a sense of what lichens are most common in the region and how much they are impacted by pollutants
Is this a question? Click here to post it to the Questions page.
Reply to this comment...
Log in to comment
In this area, lichens aren't common. Attached is a rough draft using lawn clippings to set up and test using various instruments. Don't know how well it will work-haven't tried it, yet. Getting ahold of things like centrifuge tubes can be hard. If anyone tries it, please let us know the results!
Reply to this comment...
Log in to comment
Lichen1.docx
Hopefully, this will attach.
Reply to this comment...
Log in to comment
It looks like a single beam instrument was used for this work. With a double beam instrument, there is no need for phrases like "OD435/OD415". The reference beam takes care of most issues like that. So measuring the absorbance at 435 nm is good enough. But, with single beam, it doesn't always work that way.
Reply to this comment...
Log in to comment
Login to comment.